
Development of a Validated UPLC Method for Simultaneous Analyses of 20 Ginsenosides in Various Processed Ginseng Products
Multiplexed ELISA screening assay for nine paralytic shellfish toxins in human plasma - Analyst (RSC Publishing)

DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF TRIFLUSAL IN BULK AND IN CAPSULE FORMULATION
Comparative Analysis of Protein Quantification Methods for the Rapid Determination of Protein Loading in Liposomal Formulations
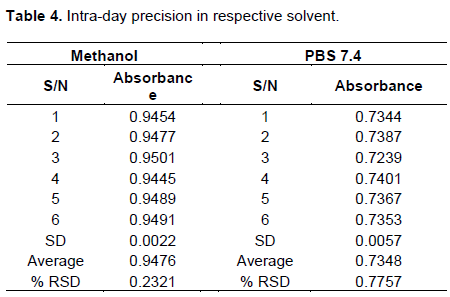
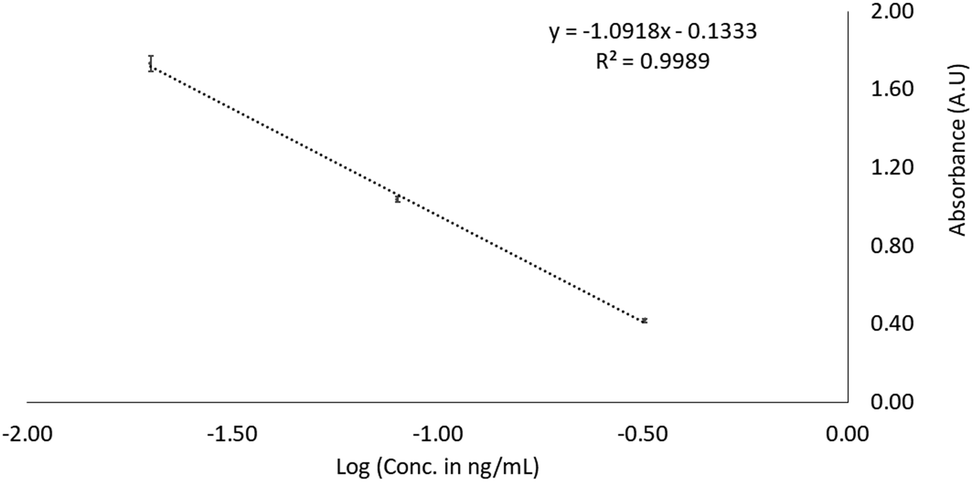
African Journal of Pharmacy and Pharmacology - development and validation of uv- spectrophotometric and rp-hplc method for the analysis of raw material and formulations of aceclofenac

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com
Original article APPLICATION OF UV-SPECTROPHOTOMETRY AND HPLC FOR DETERMINATION OF VENLAFAXINE AND ITS FOUR RELATED SUBSTANCES I
Quantitation of Verapamil and Norverapamil in Small Blood Samples From the Rat by High Performance Liquid Chromatography
Comparative Analysis of Protein Quantification Methods for the Rapid Determination of Protein Loading in Liposomal Formulations
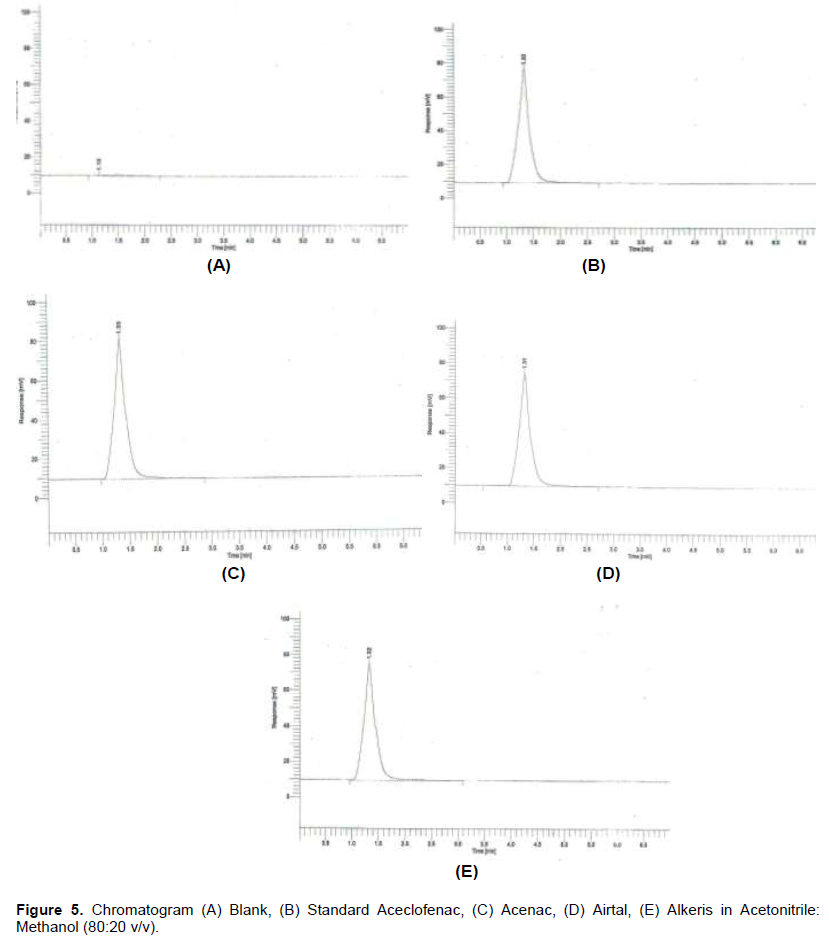
African Journal of Pharmacy and Pharmacology - development and validation of uv- spectrophotometric and rp-hplc method for the analysis of raw material and formulations of aceclofenac

PDF) Development of UV Spectroscopic Method for Nefopam and Escitalopram as INN Drugs in Tablet Dosage Form | Zakiur Rahman and Kanij Fatema - Academia.edu

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com

Estimation of measurement uncertainty and validation of RP-HPLC for simultaneous determination of five antihistamines in pharmaceutical formulations | SpringerLink

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com
A New Rapid and Sensitive Stability-Indicating UPLC Assay Method for Tolterodine Tartrate: Application in Pharmaceuticals, Human
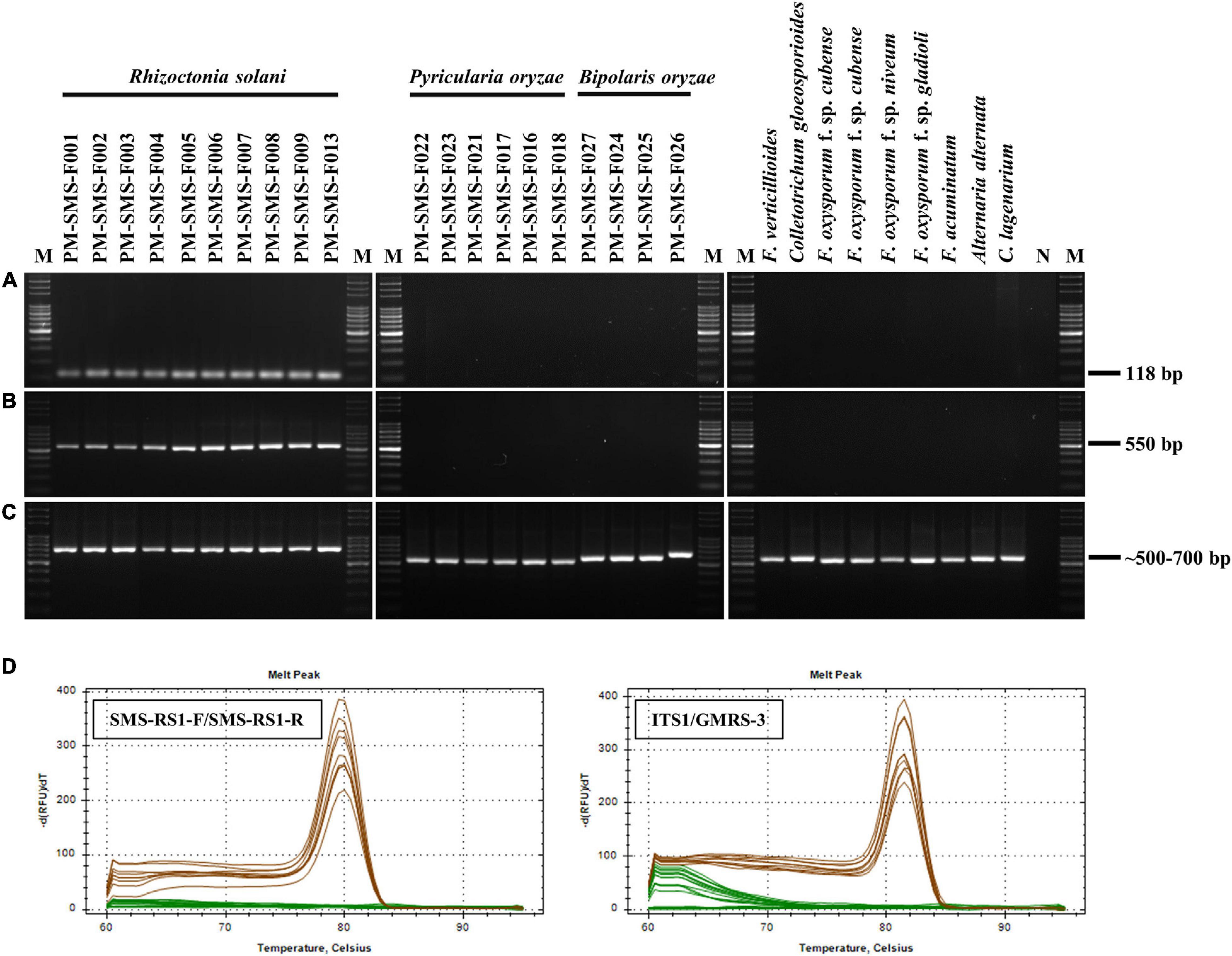
Frontiers | Molecular Detection Assays for Rapid Field-Detection of Rice Sheath Blight | Plant Science

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com
Development and validation of a stability indicating HPLC method for the simultaneous analysis of lopinavir and ritonavir in fix

Development of Micellar Electro Kinetic Chromatography for the Separation and Quantitation of L-valine, L-leucine, L-isoleucin and L-phenylalanine in Human Plasma and Comparison with HPLC - SciAlert Responsive Version



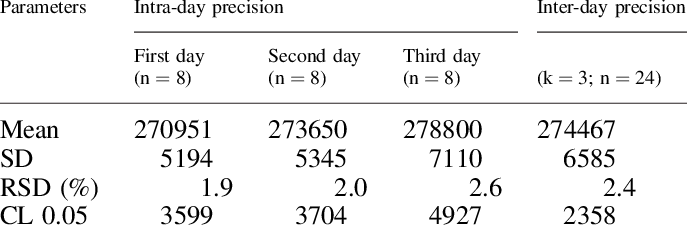


![Full text] Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-t | DDDT Full text] Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-t | DDDT](https://www.dovepress.com/cr_data/article_fulltext/s201000/201907/img/DDDT_A_201907_T0004.jpg)
